
The Block Imaging team recently had the opportunity to confer with Keith Mildenberger of Neusoft Medical Systems about all things XR-29. One of the topics he was kind enough to share about was ways that facilities can check their CT scanners for the features required to be NEMA XR-29 compliant.
We'll be sharing these insights below to help you better understand which CT units will avoid reimbursement cuts and which ones won't. AND, in case you're wondering who Keith is and why we think of him as such an authority on NEMA XR-29, you should know that he had a hand in writing the standard. So, straight from the proverbial horse's mouth: How to check your CT scanner for NEMA XR-29 compliance, in 5 steps.
Step 1: Ask Your OEM
The best advice is to ask your equipment manufacturer, specifically someone in a sales or marketing capacity. If your system has not been maintained by the manufacturer it is possible that some requirements simply have not been installed. In many cases, the manufacturer will install these free of charge, as all manufacturers have been encouraged by the FDA to implement features like Dose Check as widely as possible.
If you cannot get a clear answer from your manufacturer, there are some things you can do to make the determination yourself.
Step 2: Check for AEC
Each OEM calls their AEC features by a proprietary name. Look for the following product names associated with your scanner. If your console shows the corresponding name as enabled, your scanner should meet the AEC requirement.
- GE – Auto mA/ Smart mA
- Toshiba – SUREEXPOSURE
- Siemens – CareDose
- Philips – DoseRight
- Hitachi – Intelli EC
There should also be a way to control AEC via the control panel of your CT system. Tabs or menu options that give you access to these controls are another indicator that AEC is on your system.
It is also relatively simple to look at a study and see if you have AEC. To do this, you'll need to compare several images from the same patient scan, noting the mA per slice. If you can see that the mA is being adjusted based on the attenuation of the anatomical region being imaged, this is an indication that your system is equipped with AEC.
Step 3: Check for Dose Check
Dose Check is a NEMA standard and if you ask your manufacturer they may be willing to install it free of charge. If it is already on your system there should be a button or tab somewhere on the CT console that will allow you to access Dose Check information, set up thresholds, etc. The example below is a screen shot of the Dose Check page from a NeuViz 16 CT system. The Dose Check selection button is clearly displayed and will give the user access to Dose Alert information.
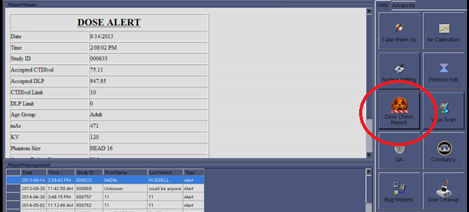
The important thing to realize about Dose Check is that it is intended to alert the technologist that there is potential to over radiate a patient BEFORE the scan is executed. This is a patient safety mechanism and the point of the NEMA XR-25 standard that defines Dose Check. There are products being offered that use the “Dose Check” name, but they can only report that a patient has been over radiated after the fact. They ARE NOT NEMA XR-29 compliant and do not provide a safety mechanism for the patient.
Step 4: Check for Reference Protocols
Generally, the standard protocols can be displayed on your control console, categorized by anatomical region. They should also be differentiated in some way to determine whether they are for adults or pediatric patients. Below is a screenshot from a NeuViz 16 CT system. A pediatric abdominal protocol is circled in red. The button is pink- color coded to identify it as a pediatric protocol.
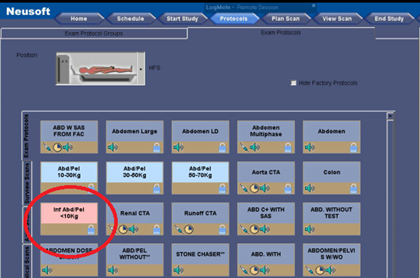
Step 5: Check for DICOM Radiation Dose Structured Reporting (RDSR)
This is the most misunderstood XR-29 requirement and the one a system in question is most likely to not have. DICOM RDSR is defined by the larger DICOM standard. For a system to be truly NEMA XR-29 compliant, it needs to be able to generate a structured report that conforms with the object definitions in the DICOM 2011 PS 3.3 standard.
DICOM RDSR is intended to allow a CT system to report patient dose information in a uniform fashion to an enterprise-wide dose reporting platform. Enterprise-wide dose reporting platforms are not common in the clinical environment yet but, as dose reporting legislation proliferates, DICOM RDSR will be a very important consideration. Laws are already in effect in California, Texas, and Connecticut.
DICOM RDSR allows your CT to interface to an enterprise-wide dose reporting product regardless of its manufacturer. This way, if you add or change a dose reporting product, there are not any costly interface requirements necessary to allow your CT to supply the requisite dose information to your new dose reporting system.
If your system has DICOM RDSR capability an RDSR file will be displayed in the patient directory. Pay special attention to the “modality” column. If you see that a file has the “SR”, or structured report, type designation this means it is a DICOM RDSR file and NEMA XR-29 compliant. Do not confuse this with the dose page that is also part of the patient directory, you can tell the difference because the “modality” designation for that file will be “CT”.
Next Steps
Depending on what you learned from completing Keith's 5-step process, you may be thinking about proceeding in a number of ways. Whether you'd like a second opinion from a field service engineer, or need to consider replacing your CT with a new or refurbished system, we can help.
.........................
Steps 1-5 courtesy of guest contributor: Keith Mildenberger
Keith has been in the diagnostic imaging business since 1983. Having held service, management, marketing, and sales positions, he has developed a broad base of experience in areas impacting the diagnostic imaging community and the patients it serves. He has been part of MITA since 2011 and involved with all aspects of NEMA XR-29, including its authorship. He is currently the CT Product Manager for Neusoft Medical Systems USA, Inc.

Chris Sharrock
Chris Sharrock is the Vice President of Healthcare Solutions at Block Imaging. Each day Chris sets out to provide the best equipment, parts, and service solutions for healthcare facilities across the world. Outside of work Chris enjoys playing in a band, and spending time at the lake with his family.






