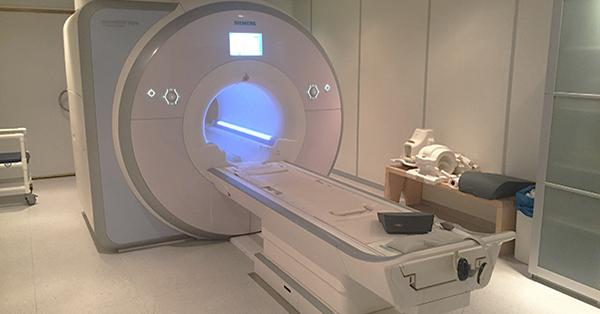
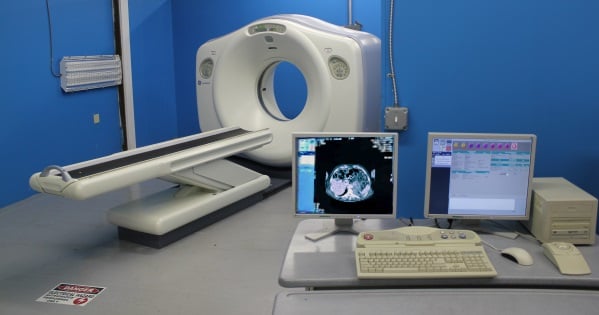
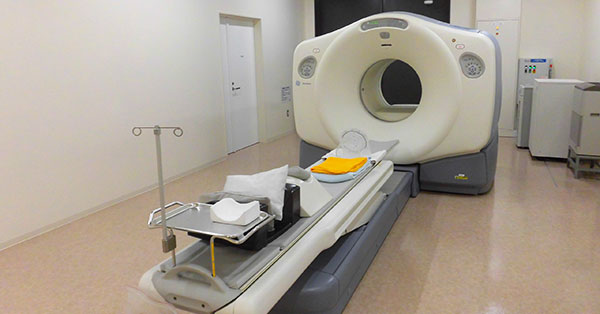
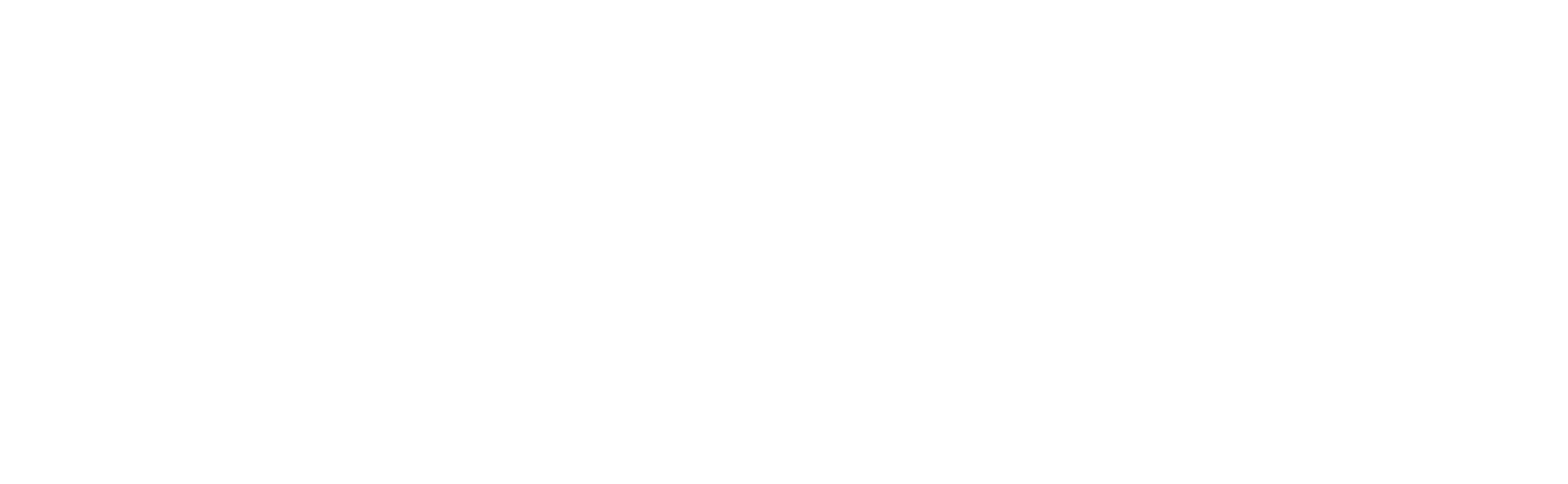So, here's a relevant, but completely hypothetical (unless you're living it right now) scenario for you: The FDA auditor is in your office, ready to inspect your X-ray imaging equipment, when you hear him say, “May I see a copy of the 2579 form for this system?”
Do you know which form he's talking about (click here if you don’t)? If you do, do you know where it is? If your imaging facility can't provide a completed FDA 2579 form for each diagnostic X-ray system in the building, you can be written up in violation of federal requirements and may be subject to associated penalties.
If you aren't sure where your form is or if you even have one, don’t panic, we'll share with you below four different ways you can get your hands on another copy of your FDA 2579.
Never heard of FDA 2579? Find out about it here.
Four Ways to Find Your FDA 2579
Contact Your Assembler
Your first and fastest bet is to call the company that installed the equipment for you, your "assembler" and request a copy. Assemblers are responsible for filling out the FDA 2579 form in quadruplicate and filing it with your state health department and the FDA itself, and leaving you a copy. In the event your copy is MIA, the Assembler should also have retained a copy in their files.
Contact Your State
You can also contact your state radiological health department to request a copy. If your original vendor isn’t available, this is the next most likely place to have a copy on file. If things were handled properly when your equipment came to your facility, one of the four copies should be in the state records for radiation-producing equipment.
Have the FDA Contacted on Your Behalf
Another option is to request that the assembler contact the FDA to obtain a copy. This may prove to be a bit more time-consuming than options one and two because the request to the FDA can only come from the assembler. The assembler will have to prove that they installed the equipment in order to obtain it.
Use the Freedom of Information Act
If none of the other options will work, you can file an FOIA request to secure a copy of the form from the FDA. This is a last resort. You don’t want to go there unless you have to, as the process can take up a significant amount of your time.
The Takeaway
We know that everyone gets busy and maintaining the paper trail on your CT scanner or digital rad room can get pushed to the back burner pretty quickly. As far as the FDA is concerned, however, it’s imperative to file the important stuff so you can find it when you need it.
If you haven't found yourself in an audit situation yet- be proactive! Make sure your paperwork is on hand. If it isn't, it's never too early to reach out to your assembler or your state health department to make sure you're ready when the auditor comes.

Trish Payne
Trish Payne is Block Imaging’s OEM and FDA Liaison. Trish is passionate about understanding the causes of challenges and working collaboratively to overcome them for the good of the imaging industry and the healthcare providers it serves. In addition to keeping Block Imaging’s work in step with industry standards, Trish is a wife, mother of two, tennis player, and world traveler.